
Cảbon is a fundamental chemical element with the atomic number 6, renowned for its unique properties and vast range of applications. This element plays a crucial role in various fields, including chemistry, biology, and industry. Understanding cảbon’s characteristics and its impact on our daily lives can provide insights into its significance.
The Atomic Structure of Cảbon:
Cảbon has an atomic number of 6, which means it has six protons in its nucleus. This atomic structure allows cảbon to form a diverse array of compounds, making it one of the most versatile elements known. Its ability to bond with other elements, particularly itself, leads to the creation of an extensive range of chemical substances.
The Unique Bonding Properties of Cảbon:
One of the most remarkable aspects of cảbon is its ability to form strong covalent bonds. Cảbon atoms can bond with each other to create chains, rings, and complex structures.
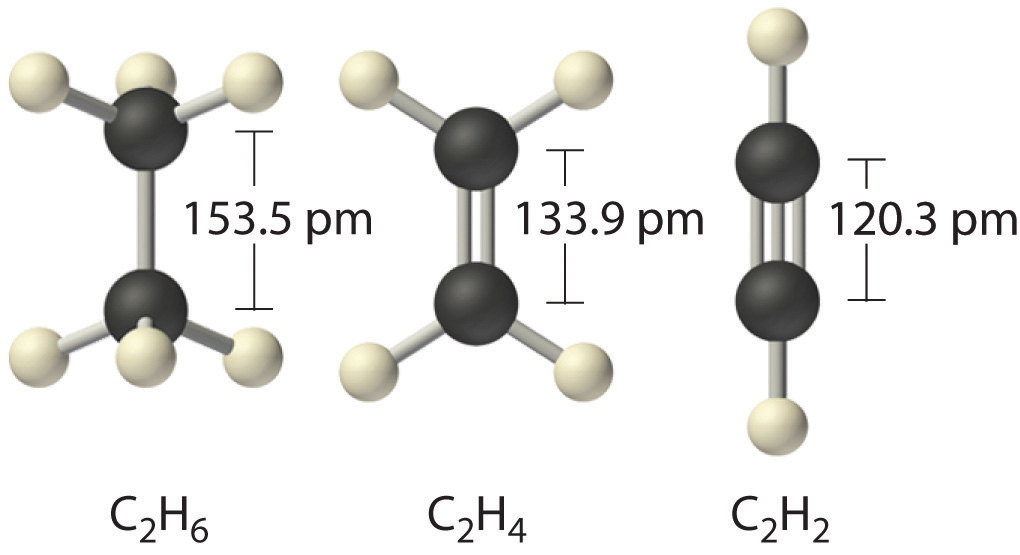
This property is responsible for the existence of a vast number of compounds, exceeding ten million known today. The versatility in bonding makes cảbon essential in organic chemistry, which focuses on compounds primarily composed of both carbon and hydrogen.
Cảbon in Organic Chemistry:
Cảbon’s role in organic chemistry is unparalleled. Organic compounds are those that contain carbon, and the study of these compounds encompasses a broad spectrum of substances.
From simple hydrocarbons like methane to complex biomolecules like DNA, cảbon is at the heart of organic chemistry. The diversity of organic compounds is attributed to the different ways in which carbon atoms can bond and interact with other elements.
Applications of Cảbon in Everyday Life:
Cảbon is not just a theoretical concept; it has practical applications that impact our daily lives. Here are some examples:
1. Materials and Industry:
Cảbon is a key component in various materials used in industries. For instance, graphite, a form of carbon, is used in lubricants, batteries, and as a moderator in nuclear reactors. Diamond, another allotrope of carbon, is valued for its hardness and is used in cutting tools and jewelry.
2. Biology and Medicine:
In biology, carbon is a fundamental building block of life. It forms the backbone of organic molecules that are crucial for biological processes. For example, carbohydrates, proteins, lipids, and nucleic acids all contain carbon. In medicine, carbon-based compounds are used in pharmaceuticals and diagnostic tools.
3. Environmental Impact:
Cảbon compounds play a significant role in environmental science. Carbon dioxide (CO₂), a compound of carbon, is a greenhouse gas that affects global climate patterns. Understanding the role of carbon in the environment helps in addressing issues related to climate change and sustainability.
The Future of Cảbon Research:
As scientific research advances, new applications and forms of carbon continue to be discovered. Nanotechnology, for example, leverages the unique properties of carbon nanotubes for innovative applications in electronics, materials science, and medicine. The study of carbon remains a dynamic field with ongoing research exploring its potential uses and impacts.
The Role of Cảbon in Energy Storage and Conversion:
Cảbon plays a pivotal role in energy storage and conversion technologies. In batteries, particularly lithium-ion batteries, carbon is used in the anodes to enhance their performance.
The high surface area and electrical conductivity of carbon materials, such as graphene and activated carbon, contribute to the efficiency and longevity of these batteries. Additionally, carbon-based materials are being researched for use in supercapacitors, which offer rapid energy storage and discharge capabilities.
The development of advanced carbon materials is crucial for improving energy storage systems, making them more efficient and sustainable for various applications, including renewable energy integration and electric vehicles.
Carbon Nanotubes and Their Applications:
Carbon nanotubes (CNTs) are cylindrical structures composed of rolled-up sheets of graphene, showcasing extraordinary mechanical, electrical, and thermal properties.
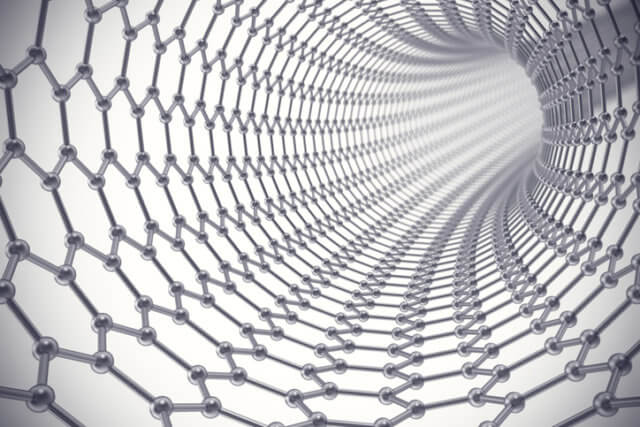
These nanomaterials exhibit tensile strength greater than steel and high electrical conductivity, making them ideal for various applications. In electronics, CNTs are used to create nanoscale transistors and conductive films, potentially leading to faster and more efficient electronic devices.
In medicine, CNTs are explored for drug delivery systems and imaging techniques due to their ability to penetrate cell membranes and their high surface area for attaching therapeutic agents. The unique properties of CNTs open new frontiers in technology and materials science, leading to innovative solutions across multiple disciplines.
The Impact of Carbon in Climate Change and Carbon Footprints:
Carbon’s role in climate change is a critical concern due to its influence on greenhouse gas emissions. Carbon dioxide (CO₂), a byproduct of burning fossil fuels and deforestation, contributes to the greenhouse effect by trapping heat in the Earth’s atmosphere. This leads to global warming and environmental changes, such as rising sea levels and altered weather patterns.
Efforts to mitigate climate change include reducing carbon emissions, transitioning to renewable energy sources, and implementing carbon capture and storage technologies. Understanding and managing our carbon footprint is essential for addressing climate change and promoting environmental sustainability.
The Significance of Carbon Allotropes in Materials Science:
Carbon exists in several allotropes, each with distinct properties and applications. Beyond graphite and diamond, other allotropes include fullerenes and graphene.
Fullerenes, such as buckyballs and carbon nanotubes, have unique molecular structures that offer potential uses in nanotechnology and materials science. Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, exhibits exceptional electrical conductivity, mechanical strength, and thermal conductivity.
These properties make graphene a promising material for various applications, including flexible electronics, advanced coatings, and high-strength composites. Research into these carbon allotropes continues to uncover new possibilities and innovations, demonstrating carbon’s versatility and importance in cutting-edge technologies.
Carbon in the Form of Synthetic Polymers:
Synthetic polymers, such as plastics, are primarily composed of carbon-based molecules. These materials are created through polymerization processes, where small monomers with carbon chains are chemically bonded to form long polymer chains.
Common examples include polyethylene, polypropylene, and polystyrene, which are used in a wide range of products from packaging to household items. The flexibility and versatility of synthetic polymers stem from the diverse ways carbon atoms can be linked together in polymer chains.
Advances in polymer chemistry have led to the development of high-performance materials like Kevlar and Teflon, which exhibit superior strength and resistance to heat and chemicals. The ongoing innovation in polymer science continues to enhance the functionality and sustainability of these materials.
Carbon in the Field of Carbon Capture and Storage (CCS):
Carbon capture and storage (CCS) is a technology designed to reduce carbon dioxide emissions from industrial processes and power plants.
The process involves capturing CO₂ emissions before they are released into the atmosphere, transporting them to storage sites, and injecting them into geological formations for long-term storage. This technology aims to mitigate the impact of greenhouse gases on climate change.
Various methods are employed for capturing CO₂, including pre-combustion, post-combustion, and oxy-fuel combustion. The effectiveness of CCS depends on the selection of suitable storage sites and the monitoring of potential leakage. CCS is considered a crucial component of strategies to achieve climate goals and reduce the global carbon footprint.
Carbon in the Context of Organic Synthesis and Drug Development:
Organic synthesis relies heavily on the versatility of carbon to create complex molecules used in pharmaceuticals and other chemical products. In drug development, carbon-based compounds are synthesized to design and optimize new medications.
The ability of carbon to form stable bonds with other elements allows chemists to build intricate molecular structures that can interact with biological targets. For instance, many antibiotics, anti-cancer drugs, and hormones are carbon-based compounds.
Advances in synthetic chemistry and computational methods have enabled researchers to explore new chemical space and design novel drugs with improved efficacy and reduced side effects. Carbon’s role in organic synthesis is fundamental to the progress of medicinal chemistry and pharmaceutical research.
The Role of Carbon in Agriculture and Soil Health:
Carbon is essential in agriculture, particularly in the context of soil health and fertility. Organic matter in soil, such as decomposed plant material and animal manure, is rich in carbon and contributes to soil structure and nutrient availability.
The presence of carbon in the form of humus helps retain moisture and improves soil aeration, which benefits plant growth. Soil carbon sequestration, the process of capturing and storing carbon in soil, is a critical strategy for mitigating climate change.
Practices such as cover cropping, reduced tillage, and organic farming enhance soil carbon storage and improve soil health. Understanding and managing soil carbon dynamics is vital for sustainable agriculture and environmental stewardship, highlighting carbon’s integral role in maintaining productive and healthy ecosystems.
FAQs About Cảbon:
1. What is the atomic number of cảbon?
The atomic number of cảbon is 6.
2. Why is cảbon important in organic chemistry?
Cảbon is important in organic chemistry because it forms a wide range of compounds by bonding with other elements, including itself, which results in a vast number of organic substances.
3. What are some common forms of cảbon?
Common forms of cảbon include graphite, diamond, and amorphous carbon.
4. How does carbon impact the environment?
Carbon compounds, such as carbon dioxide, impact the environment by contributing to climate change and affecting global temperature patterns.
5. What are some industrial uses of cảbon?
Cảbon is used in various industrial applications, including lubricants, batteries, cutting tools, and as a component in materials like carbon fiber.
6. How does research on carbon influence technology?
Research on carbon leads to advancements in technology, such as the development of carbon nanotubes, which have applications in electronics, materials science, and medicine.
Conclusion:
Cảbon, with its atomic number 6, stands out as a crucial element in both science and industry. Its ability to form a multitude of compounds and its essential role in organic chemistry highlight its significance. From everyday materials to complex biological systems, cảbon influences various aspects of our world. As research progresses, the importance of cảbon will continue to grow, shaping the future of science and technology.